Abstract
Introduction
Relapsed/refractory (R/R) AML patients continue to be a formidable clinical challenge, mainly in consideration of associated very poor outcome, with a median overall survival (OS) of less than 12 months. SCT represents the only curative option for these patients. Although, there is no standard-of-care approach which may serve as a bridge to SCT. Our study aims to investigate the effectiveness of MEC regimen as a rescue therapy for R/R AML patients by specifically addressing the CR rate, including minimal residual disease (MRD) negativity, the number of patients who subsequently underwent SCT and the presence of predictive factors of response.
Methods
Fifty-five consecutive adult AML patients were treated with MEC regimen in our Institution. In patients under 66 years old, we administered mitoxantrone 6 mg/sqm/die from day 1 to day 6, etoposide 100 mg/sqm/die from day 1 to day 6 and cytarabine 1000mg/sqm/die from day 1 to day 6, whereas in patients over 66 years old, the treatment schedule was reduced to 4 consecutive days. Data were retrospectively collected by using RedCap in accordance with Helsinki declaration and GCP. We used Kaplan-Meyer to estimate survival, and log rank to test differences in survival. Chi-squared, fisher's exact test and linear-by-linear correlation were used to test differences in proportions and distributions. Response was defined in accordance with 2017 ELN recommendations. CTCAE 4.03 was used to grade adverse events. MRD was assessed with WT1 or specific fusion transcripts.
Results
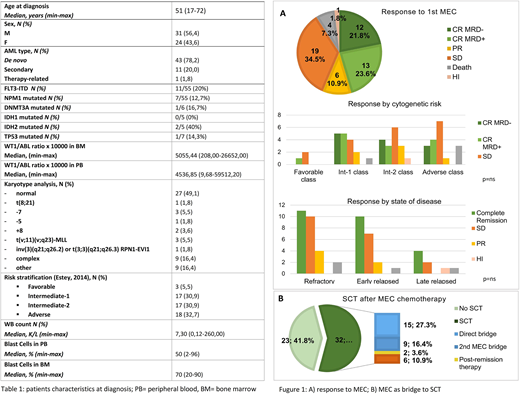
Fifty-five patients received MEC from 2008 to 2018. Age at diagnosis ranged from 17 to 72 years, with a median age of 51 years. Our set was enriched for high-risk patients. Interestingly, twenty percent of patients harbored FLT3-ITD at diagnosis (table 1). Two main groups were included: resistant AML, 28/55 patients (50,9%), and relapsed AML, 27/55 patients (49,1%). At induction, almost half of patients received "3+7" (n=25, 45,5%), while fludarabine-based regimens were administered to 14 patients (25,5%).
In our set, after MEC median duration of hospitalization was 30 days (14-78); PMN >500/mm3 was reached after 26 days (range 18-67). Fever and febrile neutropenia was the most recurrent adverse events (AE). AEs were low in grade; out of 80 graded AEs, 38 (47,5%) were grade 2, 27 (33,8%) were grade 3, 9 (11,3%) were grade 4 and only 3 events resulted in death (3,8%). E. coli was the most recurrent cause of infection (10 cases).
Overall, 25/55 patients (45,5%) achieved a complete remission (CR) after one course of MEC chemotherapy. Twelve patients (21.9%) achieved MRD negativity and 13 patients (23,6%) obtained an MRD+ CR or had no MRD test. Six patients (10,9%) had a partial response (PR) and 1 patient (1,8%)had hematological improvement (HI). Four patients (7.3%) died during post-MEC aplastic phase. Disease risk at diagnosis and R/R status did not influence the chance to obtain CR (figure 1 A). In 12 patients, a second MEC was administered. Four out of 12 patient improved their response with the 2nd MEC (2 patients obtained MRD - from MRD+ CR, 1 patient obtained PR and 1 patients obtained CR from hematological improvement).
MEC was an effective bridge to SCT, 32/55 patients (58,2%, figure 1 B), received SCT; 15/32 patients (46,9%) received SCT directly after the 1st course of MEC, 9/32 patients (28,1%) after the 2nd course of MEC and 2 patients (6,3%) after an additional course of post-remission chemotherapy. Of note, only 6 patients (18,8%), who were not responsive to MEC, underwent SCT after an alternative rescue therapy.
Median overall survival (OS) from MEC was 455 days (95% C.I. 307-602 days.); 1-year OS, 3-year OS and 5-years OS were 57,9%, 33,2% and 23,1%, respectively (std. error ± 0,067). Patients who responded to MEC (CR MRD+ or CR MRD- after 1 or 2 courses) had better OS than non-responders (median OS 1389 vs 160 days, p=.003). Stepwise multiple logistic regression analysis with COX-HR model established that pre-MEC R/R status, diagnosis class risk, response to one or two courses of MEC, and SCT were independent predictors of survival in the optimal model.
Conclusions
Taken together, our data indicate that MEC is an effective salvage regimen with affordable toxicity, and gives a high chance to obtain CR. MEC is particularly useful as a bridge to SCT, and has to be considered as a rescue therapy whenever a clinical trial is not available.
*GM and AT equally contributed
Martinelli:Ariad/incyte: Consultancy; Pfizer: Consultancy; Celgene: Consultancy; Amgen: Consultancy; Janssen: Consultancy; Roche: Consultancy. Cavo:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.